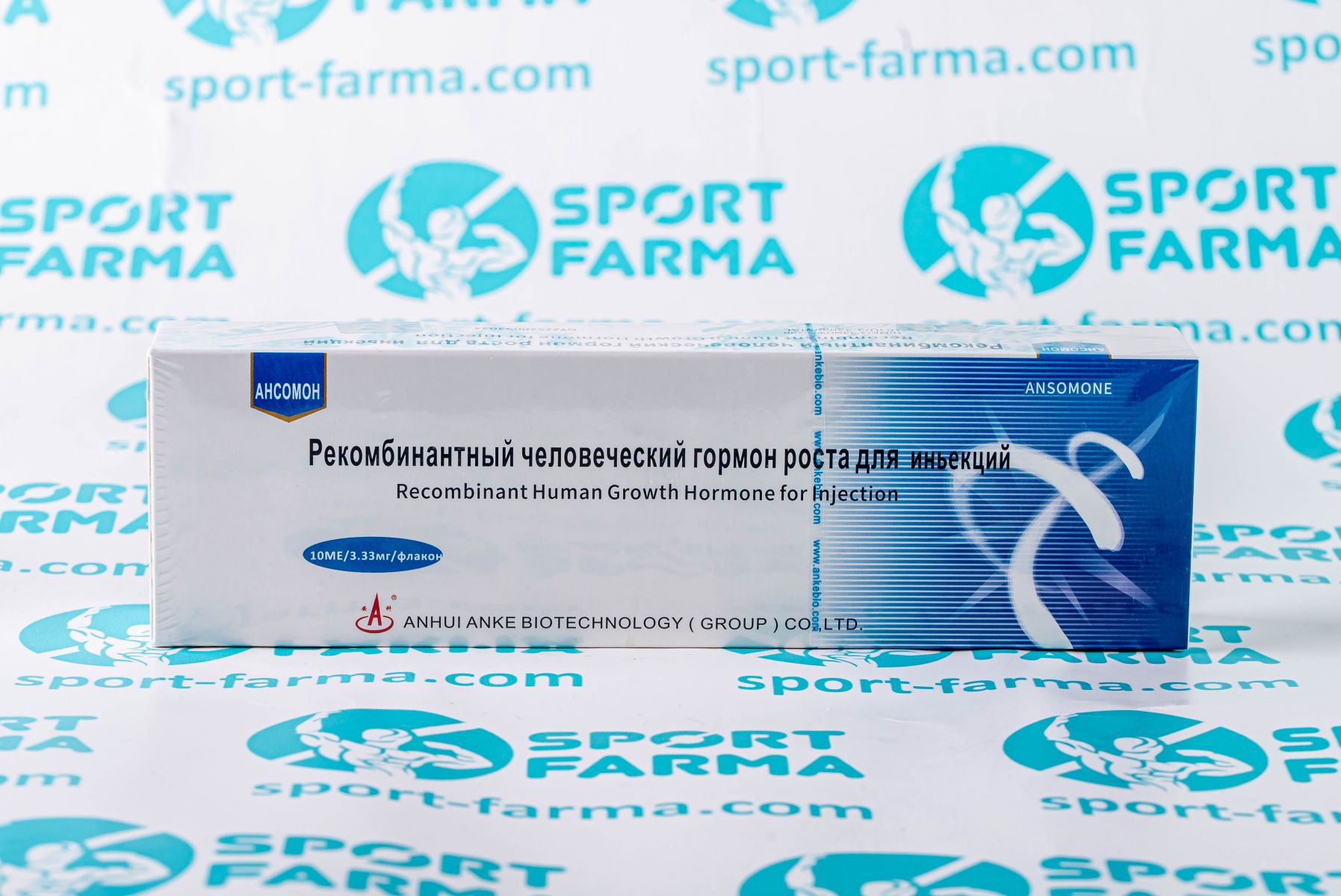
Description
Ansomone is a medical product produced by the Chinese company Anhui Anke Biotechnology, which specializes in the development and production of growth hormone. The unique composition of Ansomone contains identical natural recombinant human growth hormone with one hundred and ninety-one amino acids.
Indications:
Ansomone is widely used in medical practice, stimulating linear growth and increasing growth rates in children who lack adequate endogenous growth hormone. Also, the drug is used for renal failure or Turner's syndrome, for the effective treatment of various injuries and burns. In addition, Ansomone has a rejuvenating effect, promotes an increase in muscle mass and rapid fat burning.
The action of the drug Ansomon:
- Significant reduction in body fat
- Increase in lean body weight
- Strengthening the immune system
- Improving the physiological state of the whole organism
- Increased libido
- Improved bone strength
- Increased energy levels, uplifting mood
- Fast healing of any wounds and burns
- Stimulation and enhancement of cell growth
By purchasing Ansomon, you get increased mental, physical and creative abilities, a good mood, as well as the opportunity to get a beautiful body even faster, because with Ansomon fat is burned and muscle mass grows many times faster! The use of Ansomon is extremely effective with regular physical activity, for example, during physical exercises, such as exercising in the gym.
Mode of application:
Ansomon is administered by subcutaneous and intramuscular injections and the route of administration does not affect the effect and pharmacological result.The maximum concentration of the drug is reached after 3-5 hours. Excretion of growth hormone from the human body occurs through the kidneys and liver with a half-life of 2-3 hours.


Reviews
There are no reviews yet.